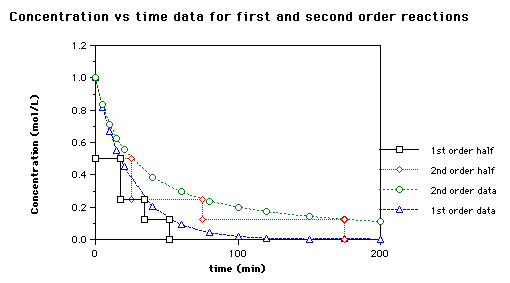
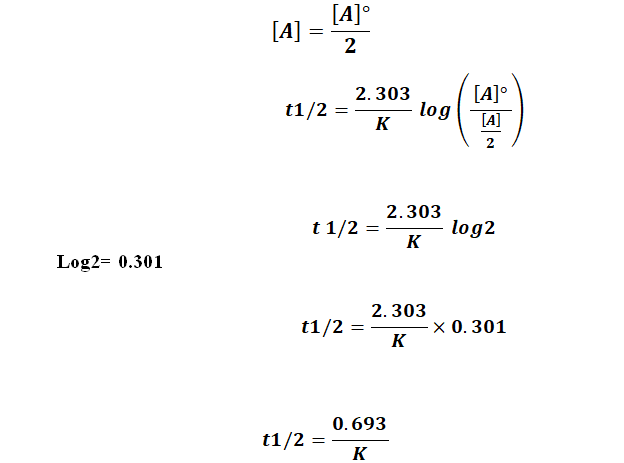half life formula for first order reaction
The formula for the half-life of different reactions is given below. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant.
Solved Please Help With Q1 2 3 4 5 6 7 8 9 Amp Chegg Com
The half-life of a drug can be determined using the.

. The half-life of a first-order reaction is given as t 12 0693k. Where The half-life of a reaction is referred to as t 12. Half Life Calculator first order reaction input the equations calculated rate constant.
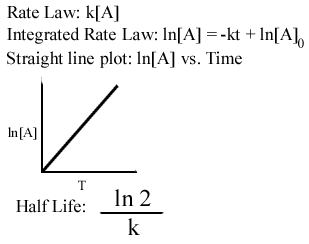
First Order Reactions. The half-life period of reaction is the time needed for the reactant concentration to fall to one half of its initial value. Half Life period of first order reaction is the time required for 50 percent completion of the reaction and is represented as t 05 ln 2 K h or Half Life Period ln 2 Rate Constant.
For first-order reactions the relationship between the reaction half-life and the reaction rate constant is given by the expression. The half-life t12 is the time it takes for the plasma concentration of a drug or the amount of drug in the body to be reduced by 50. We can write the rate expression as rate -dBdt and the rate law as rate kBb.
The unit of rate constant k for First-order reactions is sec -1. The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. T 12 0693k Where t 12 denotes the half-life of the.
After around 15 minutes about half of the popcorn has been consumed. By rearranging the above equation the half-life of a first-order reaction can be obtained to be t_12frac2303klogleft 2 right t_12frac0693k The Half-Life of a. The half-life of a second-order reaction is given by the formula 1kR 0.
Since the reaction order is second the formula for t12 k-1A o-1. The final concentration is 1167 M. This widget calculates the half life of a reactant in a.
In a first-order reaction the rate constant does. The rate equation for first order reaction is k t2303log A tA 0. Suppose we have a first order reaction of the form B.
The half-life of a first-order reaction does not depend upon the. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. The half-life period of a first-order reaction is given by t12 0693k.
The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k For the first-order reaction the half-life is defined as t12 0693k. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.
Most significantly this demonstrates that. The half-life of a first-order reaction. So here is your half-life for a first order reaction.
For a zero-order reaction the half-life equation is given as. The half-life formula for a reaction depends upon the order of a reaction. The remainder of the popcorn is consumed till the conclusion of the film.
Added Dec 9 2011 by ebola3 in Chemistry. Since the whole is 100 the first half-life would drop to 50 and then to 25. For a first zero order reaction the.

Elimination Rate Constant An Overview Sciencedirect Topics
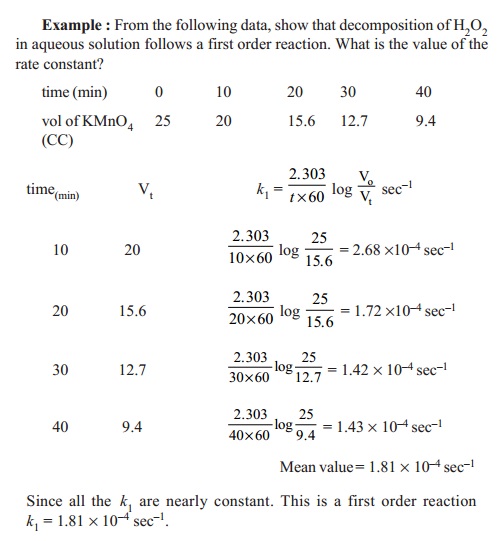
Rate Equation For First Order Reactions

E Lifes Exercise Calculation Of Kinetic Rate Of Reaction First Order Second Order Zero Order Half Life Time

In The First Order Reaction 10 Of The Reactant Is Consumed In 25 Minutes Calculate I The Half Life Period Of The Reaction Ii The Time Required For Completing 87 5 Of The Reaction From Chemistry Chemical

Half Life Of First Order Reaction Youtube
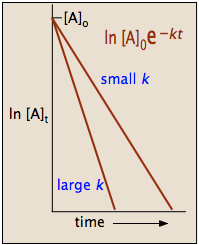
2 3 First Order Reactions Chemistry Libretexts
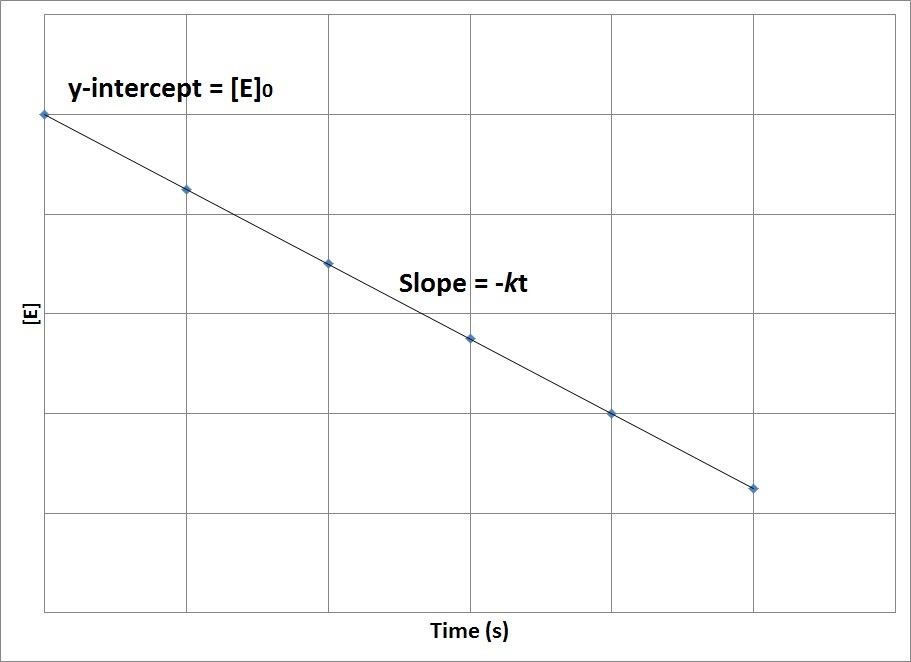
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition

Zero Order Reaction Definition Examples Formula
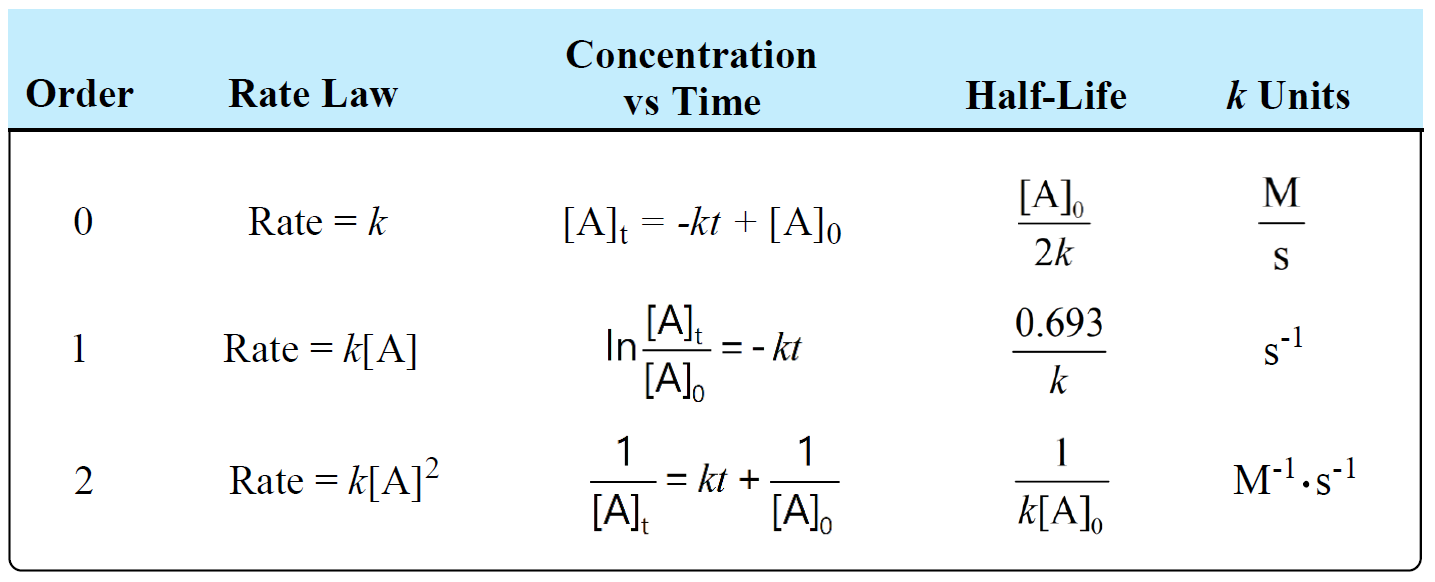
Units Of Rate Constant K Chemistry Steps

What Formula Can Be Used To Determine The Half Life Chegg Com
Learn Chemistry Tutorials Kinetics Tutorial

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1

E Lifes Exercise Calculation Of Kinetic Rate Of Reaction First Order Second Order Zero Order Half Life Time

First Order Reactions Chemical Kinetics Free Study Material

Cbse Class 12 Science Answered